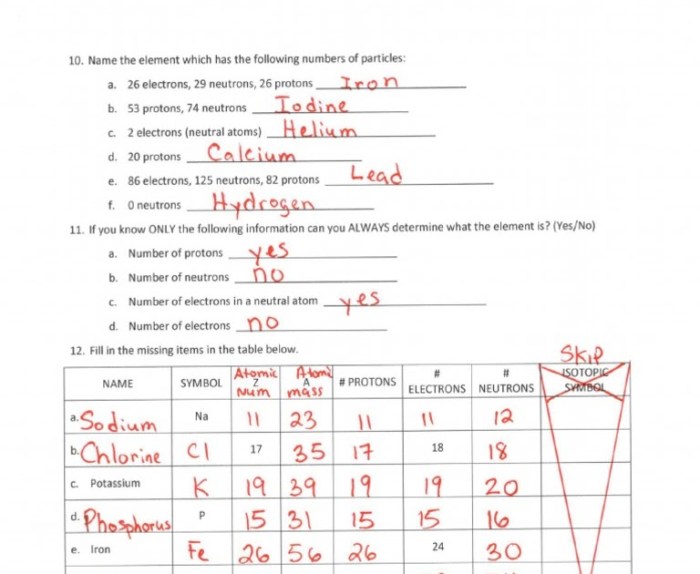
Embark on a journey into the fundamental building blocks of the universe with our comprehensive Introduction to Atoms Worksheet Answer Key PDF. This guide provides a thorough exploration of atomic structure, properties, and applications, equipping you with a deep understanding of the microscopic world.
Delving into the intricacies of atoms, we uncover their basic structure, composed of a nucleus harboring protons and neutrons, surrounded by a cloud of orbiting electrons. We delve into the arrangement of electrons in energy levels and electron shells, elucidating the relationship between the number of protons, electrons, and neutrons in an atom.
1. Definition of Atoms
Atoms are the fundamental building blocks of matter. They are the smallest units of an element that can exist independently and retain the element’s chemical properties.
Atoms are composed of a nucleus, which contains protons and neutrons, and electrons, which orbit the nucleus. The number of protons in an atom determines its atomic number, which identifies the element. The number of electrons in an atom determines its chemical properties.
2. Atomic Structure
Nucleus
The nucleus is the central part of an atom, and it contains protons and neutrons. Protons are positively charged particles, and neutrons are neutral particles.
Electrons
Electrons are negatively charged particles that orbit the nucleus. They are arranged in energy levels, with each level containing a specific number of electrons.
Relationship between Protons, Electrons, and Neutrons
The number of protons in an atom is equal to the number of electrons in an atom. The number of neutrons in an atom can vary, and it determines the isotope of the element.
3. Atomic Properties
Atomic Mass
The atomic mass of an atom is the sum of the masses of its protons and neutrons.
Atomic Number
The atomic number of an atom is the number of protons in its nucleus.
Electronegativity, Introduction to atoms worksheet answer key pdf
Electronegativity is a measure of an atom’s ability to attract electrons.
4. Chemical Bonding: Introduction To Atoms Worksheet Answer Key Pdf
Types of Chemical Bonds
- Ionic bonds
- Covalent bonds
- Metallic bonds
Factors Determining Bond Type
The type of chemical bond that forms between atoms is determined by the electronegativity of the atoms involved.
Role of Electron Configuration
The electron configuration of an atom determines its chemical properties and the type of bonds it can form.
5. Applications of Atomic Theory
Chemistry
Atomic theory is used to explain the chemical properties of elements and compounds.
Physics
Atomic theory is used to explain the structure of atoms and the behavior of matter.
Materials Science
Atomic theory is used to design and develop new materials with specific properties.
FAQ Corner
What is the significance of atomic number?
Atomic number determines the element’s identity and its position on the periodic table.
How does electronegativity influence chemical bonding?
Electronegativity affects the distribution of electrons in a bond, determining the type and strength of the bond formed.